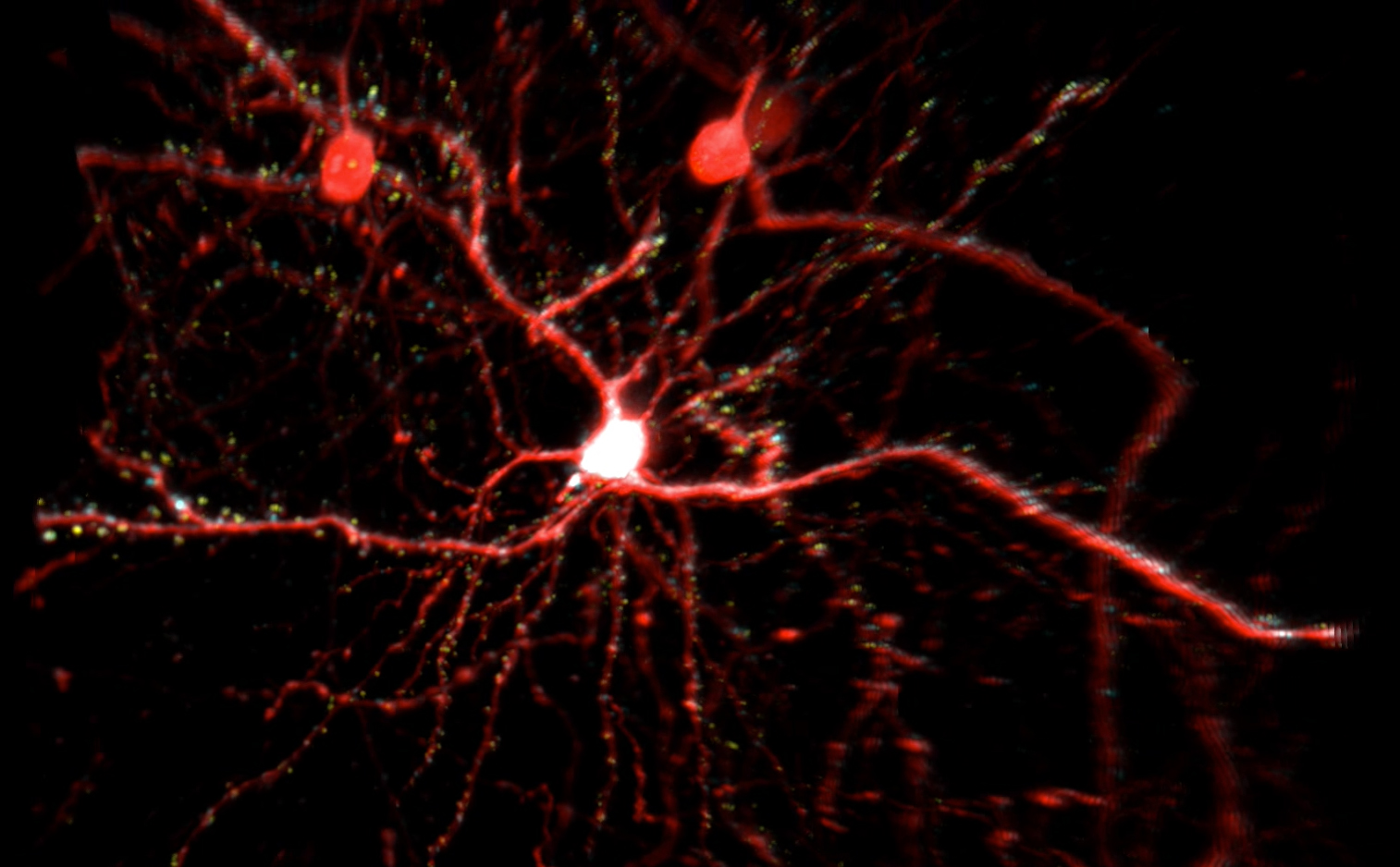
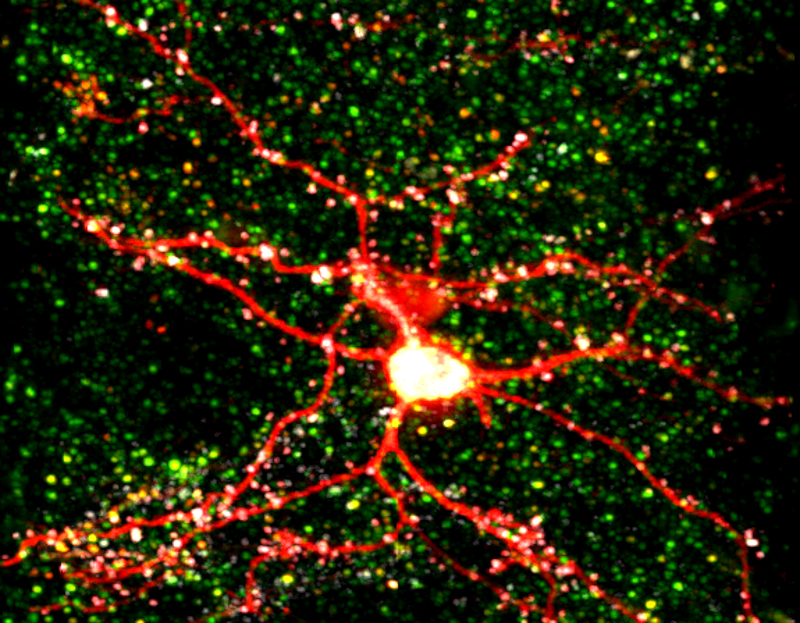
Many CPGs are capable of modifying neuronal structure, suggesting that neuronal structure can be modified by activity even in the adult brain. To investigate the molecular mechanisms underlying structural plasticity in the mammalian brain we have a long-standing collaboration with Peter So’s lab in the Department of Mechanical Engineering at MIT to develop multi-photon microscopy for large volume, high resolution imaging of dendritic arbor and synaptic structural dynamics in vivo. Using this system we have imaged and reconstructed the dendritic trees and axon collaterals of neurons in visual cortex of thy1-EGFP transgenic mice. These transgenic mice express EGFP in a random subset of neurons sparsely distributed within the superficial cortical layers that are optically accessible through surgically implanted cranial windows. The images show an abundance of detail, with the basal and apical dendrites instantly recognizable and spines clearly visible. This enables dendritic branch dynamics in individual neurons to be examined over several months. We were the first to reveal that dendritic arbor remodeling in the adult is restricted to inhibitory interneurons (Lee et al., PLoS Biology 2006), that interneuron remodeling is not a feature determined by cell lineage, but rather is imposed by the laminar cortical circuitry (Lee et al., PNAS 2008), and is elicited by sensory experience in an input-specific manner so as to facilitate or attenuate experience-dependent plasticity (Chen et al, Nature Neuroscience 2011). Further we showed that inhibitory dendrite remodeling is a common element of structural plasticity mechanisms built into the neocortical module (Chen et al., J. Neurosci. 2011).
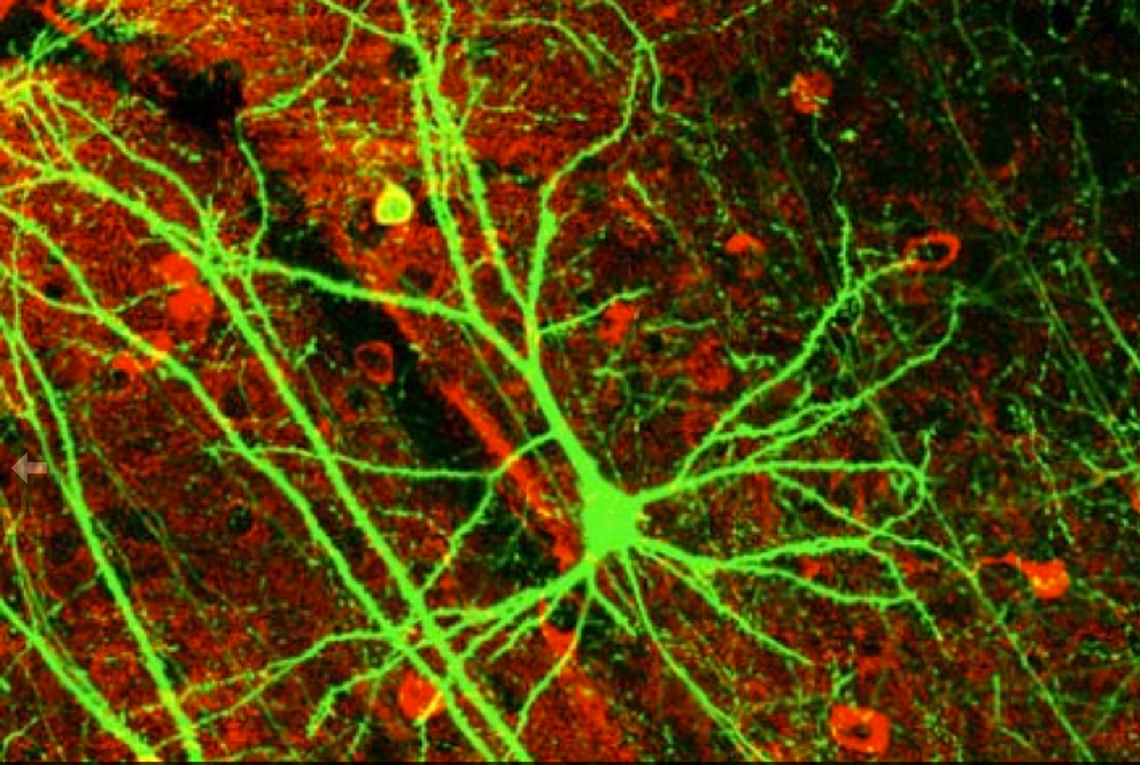
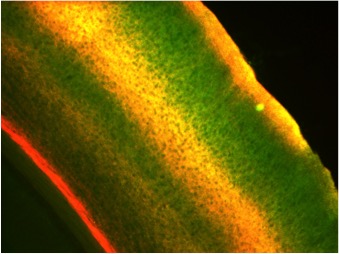
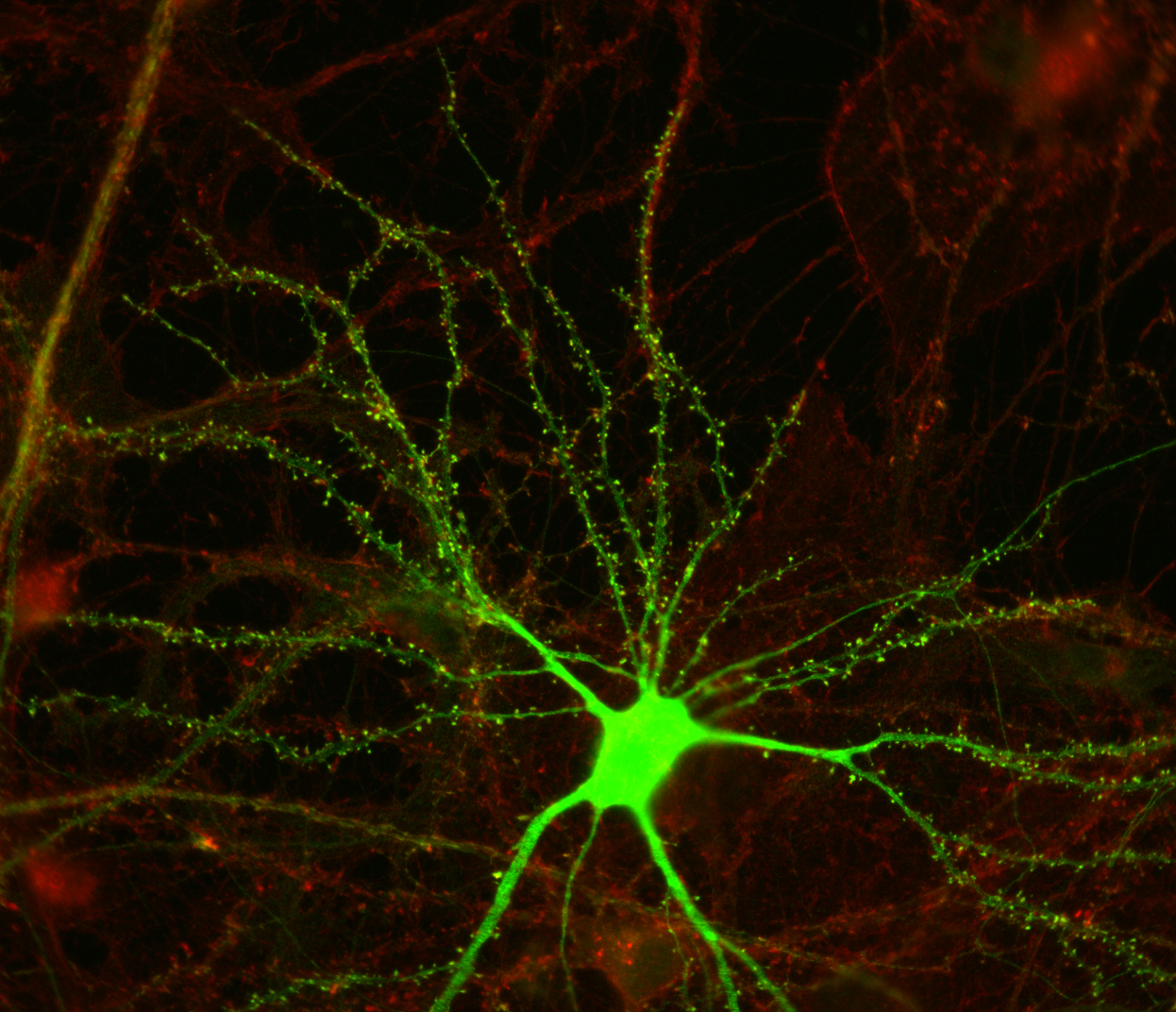
Once interneurons were recognized as key players in activity-dependent circuit plasticity, a major obstacle to addressing questions regarding inhibitory synapse plasticity as well as how it may be coordinated with excitatory inputs at the dendritic level, has been the inability to visualize inhibitory synapses in vivo. We therefore expanded our high-resolution large volume imaging capabilities with incorporation of two-color multiphoton microscopy to simultaneously monitor both inhibitory synapse and dendritic spine remodeling across the entire dendritic arbor of cortical L2/3 pyramidal neurons in vivo during normal and altered sensory experience. This has allowed us to address, for the first time, fundamental questions about the interplay between excitatory and inhibitory synaptic transmission during normal adult brain function as well as experience-dependent plasticity (Chen et al., Neuron 2012). Currently we are working to integrate additional colors into our imaging set up to enable simultaneous tracking of multiple synaptic and cellular components. We are also interested in integrating Ca+2 imaging with our structural markers.
Molecular Mechanism of Inhibitory Synaptic Rearrangements
Work from our lab and others (Cane et al., 2014; Chen et al., Neuron 2012; Kelsch et al., 2008; van Versendaal et al., 2012), has shown that both excitatory and inhibitory synapses are extremely plastic in vivo. While many of the molecular players that underlie the formation of excitatory synapses are well characterized, little is known about the molecular regulation of inhibitory synapses. My project uses molecular biology techniques as well as multi-color two photon microscopy to probe specific interactions that regulate the formation and elimination of inhibitory synapses in vivo.
In Vivo Two-Photon Imaging of Dendritic and Synaptic Structural Changes during Early Development
The ultimate structure and function of our brains are directly influenced by our experiences and interactions with the world around us. The critical period for ocular dominance plasticity is a time during development where a change in visual input to the visual cortex can cause profound changes in the connectivity of this area of the brain. For several years, our lab has developed techniques to image the entire dendritic arbors of neurons with synaptic resolution over time. In the adult, we have studied the plasticity of various types of neurons and characterized the dynamics of this plasticity during normal experience and during experimental manipulation. My project makes use of the genetic labeling and high resolution imaging techniques developed in the lab to investigate the formation of inhibitory and excitatory connections and their activity-dependent selection during the cortical critical period for ocular dominance plasticity in mice.
Mapping specificity of afferent inputs distribution across L2/3 pyramidal cell arbors
Surprisingly, we know little at the single cell level regarding the distribution of particular types of inputs onto any given cortical cell type. What is the cortical vs subcortical input source of excitatory synapses located across the dendritic arbor, and do their synaptic dynamics differ depending on this source? What is the specific interneuron type targeting inhibitory synapses located across the dendritic arbor, and do their synaptic dynamics differ depending on this source? Do inhibitory shaft synapses receive different inputs than inhibitory spine synapses? Their very different kinetics and response to visual deprivation, suggest that may be the case (Chen et al., Neuron 2012). Are inhibitory synapses receiving specific types of afferent inputs more likely to exhibit coordinated dynamics with excitatory synapses? My project uses in vivo multicolor two-photon microscopy and transgenic mouse lines to identify the afferent sources to synapses at different dendritic locales, determining whether synapses targeted by certain afferents are less or more dynamic, and whether their dynamics are coordinated.
Experience Dependent Stabilization of Synapses in Vivo
We find that in the cpg15 knockout mouse (cpg15 KO) there is abnormal postnatal development of excitatory connectivity in the hippocampus (Fujino et al., Genes & Dev. 2011), as well as visual cortex (Picard et al., J. Neurosci. 2014). In the dentate gyrus of the hippocampus as many as 30% of spines initially lack synapses. Chronic in vivo imaging of cortical pyramidal neurons through a cranial window shows that while dendritic spine dynamics in cpg15 KO mice are comparable to controls, fewer of the dynamic events in these mice are stabilized, and thus favor persistent spine loss (Fujino et al., Genes & Dev. 2011). These results suggest that the in vivo developmental deficits in the cpg15 KO mouse derive from lack of a synaptic stabilization signal. My project makes use of new in vivo synaptic labeling methods and multi-color two-photon microscopy to examine how CPG15 regulates synapse stabilization in vivo and explores whether CPG15 could link experience driven neuronal activity and new synapse stabilization.
In Vivo two Photon Imaging of Modulatory Cortical Afferent Inputs
Widespread projections of neuromodulatory neurons influence the remodeling of local neural circuits and modify their response to stimuli. Despite the critical importance of modulatory cortical afferents on plasticity, little is known about their connections in the cortex at the cellular level. I will utilize multiphoton in vivo microscopy to characterize the distribution of modulatory afferent inputs to the cortex and how they are associated with structural plasticity. By monitoring the interaction between defined modulatory inputs and local neural synaptic components we can begin to address the local influence of neuromodulators on structural plasticity of inhibitory and excitatory synapses in sensory cortex.